The Remarkable Properties of Titanium Rods in Medical Applications
Unmatched Biocompatibility: A Synergy with Human Physiology
One of the most compelling reasons for choosing titanium pole therapeutic inserts is their uncommon biocompatibility. This interesting property guarantees that the human body acknowledges the embed with negligible hazard of dismissal or unfavorable responses. The biocompatibility of titanium stems from its capacity to shape a steady oxide layer on its surface when uncovered to oxygen. This layer acts as a defensive obstruction, anticipating the discharge of metal particles into the encompassing tissues and diminishing the probability of provocative reactions.
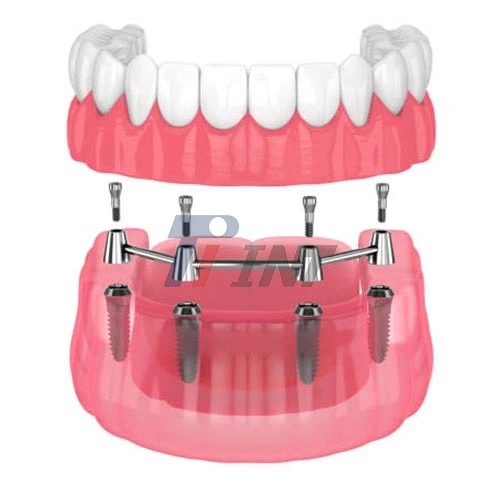
Furthermore, the biocompatibility of titanium rods extends beyond mere tolerance by the body. Research has shown that titanium implants can actually promote osseointegration – the process by which bone cells attach directly to the implant surface. This remarkable property enhances the stability and longevity of the implant, making titanium rods an excellent choice for orthopedic and dental applications where a strong bond between the implant and bone tissue is crucial.
Lightweight yet Durable: The Perfect Balance for Medical Devices
Another significant advantage of titanium rod medical implants is their impressive strength-to-weight ratio. Titanium boasts a density of approximately 4.5 g/cm³, which is considerably lower than that of stainless steel (around 8 g/cm³) or cobalt-chromium alloys (about 8.5 g/cm³) – materials that have traditionally been used in medical implants. This lower density translates to lighter implants, which can significantly reduce the burden on surrounding tissues and improve patient comfort.
Despite their lightweight nature, titanium rods exhibit remarkable strength and durability. For instance, Grade 5 titanium (Ti6Al4V ELI), commonly used in medical implants, has a tensile strength of 860 MPa and a yield strength of 795 MPa. These values indicate the material's ability to withstand substantial stress without permanent deformation or failure. The combination of low weight and high strength makes titanium rods particularly suitable for load-bearing applications such as spinal fusion rods, hip stems, and dental implants.
Corrosion Resistance: Ensuring Long-Term Implant Integrity
The human body presents a challenging environment for any implanted material. Exposure to bodily fluids, which contain various corrosive elements, can lead to the degradation of many metals over time. However, titanium rod medical implants excel in this aspect due to their superior corrosion resistance.
The corrosion resistance of titanium is attributed to the spontaneous formation of a stable, adherent oxide film on its surface when exposed to oxygen. This passive layer, primarily composed of titanium dioxide (TiO2), acts as a protective barrier against further oxidation and corrosion. The stability of this oxide layer in physiological conditions ensures that titanium implants maintain their structural integrity and functionality over extended periods.
Moreover, the corrosion resistance of titanium rods contributes to their overall biocompatibility. By minimizing the release of metal ions into the surrounding tissues, the risk of adverse reactions or local tissue discoloration (metallosis) is significantly reduced. This property is particularly crucial for long-term implants, where sustained performance and safety are paramount.
Advanced Manufacturing Techniques for Titanium Rod Medical Implants
Precision Engineering: Customization for Optimal Patient Outcomes
The versatility of titanium as a material for medical implants is further enhanced by advanced manufacturing techniques that allow for precise customization. Titanium rods can be fabricated in a wide range of diameters, typically from 3mm to 100mm, catering to various medical applications. The length of these rods is also highly customizable, with some manufacturers offering rods up to 6 meters in length, which can then be cut to the exact specifications required for each patient.
This level of customization is crucial in medical implants, where a perfect fit can significantly impact the success of the procedure and the patient's recovery. For instance, in spinal fusion surgeries, the ability to tailor the length and diameter of titanium rods ensures optimal spinal alignment and stability. Similarly, in orthopedic applications such as intramedullary nailing, customized titanium rods can provide better fixation and support for fractured bones.
Surface Finishing: Enhancing Performance and Integration
The surface characteristics of titanium rod medical implants play a crucial role in their performance and integration with the surrounding tissues. Manufacturers offer various surface finish options, each tailored to specific medical applications:
- Polished Surface: A smooth, polished finish is often preferred for articulating surfaces in joint replacements. This finish minimizes friction and wear, potentially extending the lifespan of the implant.
- Sandblasted Surface: A rougher, sandblasted surface can enhance osseointegration by providing a larger surface area for bone cells to adhere to. This finish is particularly beneficial in dental implants and certain orthopedic applications.
- Machined Surface: A machined finish offers a balance between smoothness and texture, suitable for applications where moderate tissue integration is desired.
Quality Assurance: Meeting International Standards
The production of titanium rod medical implants is subject to stringent quality control measures to ensure safety and efficacy. Reputable manufacturers adhere to international standards and certifications, including:
- ISO 9001:2015: This certification ensures that the manufacturer follows a quality management system that meets customer and regulatory requirements.
- ISO 13485:2016: Specifically tailored for medical devices, this standard demonstrates the manufacturer's ability to provide medical devices and related services that consistently meet customer and regulatory requirements.
- CE Certification: This marking indicates that the product complies with the essential requirements of relevant European health, safety, and environmental protection legislation.
Future Prospects and Innovations in Titanium Rod Medical Implants
Advancements in Material Science: Enhancing Titanium's Properties
The field of titanium rod medical implants continues to evolve, driven by ongoing research and technological advancements. One area of focus is the development of new titanium alloys with enhanced properties. For instance, beta-titanium alloys are being explored for their lower elastic modulus, which more closely matches that of human bone. This property could potentially reduce stress shielding – a phenomenon where the implant bears most of the load, leading to bone resorption around the implant.
Another promising avenue is the development of porous titanium structures. These structures mimic the architecture of natural bone, potentially improving osseointegration and reducing the risk of implant loosening over time. Advanced manufacturing techniques such as 3D printing are enabling the creation of these complex, porous titanium structures with unprecedented precision.
Surface Modifications: Enhancing Bioactivity and Functionality
Research is also focusing on innovative surface modifications for titanium rod medical implants. These modifications aim to enhance the bioactivity of the implant surface, promoting faster and more robust tissue integration. Some promising approaches include:
- Hydroxyapatite Coating: Applying a layer of hydroxyapatite, a mineral found naturally in bone, to the titanium surface can enhance bone formation around the implant.
- Nanostructured Surfaces: Creating nanoscale features on the implant surface can mimic the natural extracellular matrix, potentially improving cell adhesion and proliferation.
- Antimicrobial Coatings: Developing surfaces with inherent antimicrobial properties could reduce the risk of implant-associated infections, a significant concern in orthopedic and dental implantology.
Integrating Smart Technologies: The Future of Implantable Devices
Looking ahead, the integration of smart technologies with titanium rod medical implants presents exciting possibilities. Concepts under exploration include:
- Sensor-Equipped Implants: Titanium rods with embedded sensors could provide real-time data on implant performance, bone healing progress, or early signs of complications.
- Drug-Eluting Implants: Titanium rods could be designed to slowly release medications, such as antibiotics or growth factors, to promote healing or prevent infections.
- Shape-Memory Alloys: Developing titanium alloys with shape-memory properties could allow for minimally invasive implantation procedures and adaptive implant behavior in response to physiological changes.
Conclusion
The choice of titanium rods for medical implants is supported by a wealth of scientific evidence and clinical success. Their unique combination of biocompatibility, strength, durability, and corrosion resistance makes them an ideal material for a wide range of medical applications. As manufacturing techniques continue to advance and new innovations emerge, the future of titanium rod medical implants looks brighter than ever.
If you're interested in learning more about titanium rod medical implants or exploring customized solutions for your specific needs, we invite you to reach out to our team of experts. Contact us at export@tiint.com for more information on our range of high-quality titanium products and how they can benefit your medical practice or research.










 2025-07-04 14:13:19
2025-07-04 14:13:19


_1753427753918.webp)
_1751957234428.webp)
_1750209732185.webp)



