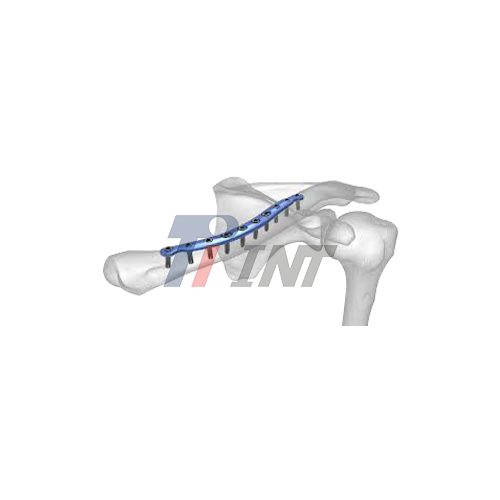
As orthopedic surgery develops and the needs for trauma surgery grow more complex, there is an increasing need for premium clavicle titanium plates. With its remarkable biocompatibility, strength, and precise engineering, China has become a worldwide leader in the production of these vital medical devices. Strict certification procedures, state-of-the-art technology, and an unshakable dedication to quality standards have allowed leading Chinese manufacturers to establish themselves. These vendors provide complete solutions for bone healing applications, internal fixation systems, and clavicle fracture care. Chinese suppliers are becoming more and more appealing to international medical device businesses and healthcare providers looking for dependable titanium implant solutions due to their affordable pricing, sophisticated production skills, and strict quality control.
|
|
|
Leading Chinese Manufacturers of Clavicle Titanium Plates
Baoji INT Medical Titanium Co., Ltd.
Since 2003, Baoji INT Medical Titanium Co., Ltd. has been a leading manufacturer of medical titanium materials. The firm was founded by Mr. Zhan Wenge, who has over 30 years of experience in the titanium sector and has mastered state-of-the-art titanium processing technology. Their extensive product line consists of rods, wires, plates, precision forged parts, and pure titanium and Ti6Al4V ELI titanium alloy materials in a range of requirements, including the clavicle titanium plate. The firm ensures that its surgical plates fulfill worldwide requirements for trauma surgery applications by maintaining ISO 13485 certification in addition to FDA and CE compliance.
More than 500 tons of medical-grade titanium materials may be produced there each year, and they have substantial export potential to supply orthopedic device manufacturers across the world. The founding of Shaanxi Stand Biotechnology Co., Ltd. shows their dedication to turning scientific discoveries into useful medical solutions, especially in the areas of locking plate systems and precision die forging for metal joint components.
Shanghai Putailai New Energy Technology Co., Ltd.
Shanghai Putailai has expanded into the medical titanium market by using their knowledge of materials science to create high-performing clavicle fixation plates. They have state-of-the-art production facilities in Shanghai's industrial center that can create the intricate geometries needed for applications including minimally invasive surgery. Comprehensive testing procedures for biomechanical strength and fracture stabilization performance are part of their quality management system.
With 200,000 titanium implants produced annually, the firm supplies major medical equipment manufacturers in North America and Europe. Their expenditures in R&D are concentrated on surface treatment technologies that improve bone healing and shorten recovery periods after surgery. Finite element analysis, material characterisation, and custom plate design for particular patient anatomy are examples of technical support services. With a strong presence in trauma surgery and fracture treatment applications, the market is present in 25 countries.
Xi'an Saite Metal Materials Development Co., Ltd.
Xi'an Saite is a titanium alloy processing specialist with a focus on orthopedic applications, such as devices for treating clavicle fractures. They can manufacture titanium plates in both conventional and customized forms, facilitating fracture reduction and plate osteosynthesis processes. The firm ensures that its products fulfill strict biocompatibility criteria by maintaining ISO 5832-3 and ASTM F136 certifications. A total of 150 tons of completed medical titanium goods may be produced annually, with 70% going to export markets.
Among its technological advantages are unique heat treatment techniques that maximize mechanical qualities for applications involving bone fixation. Prominent OEM/ODM manufacturers that cater to the shoulder injury treatment industry are among the clientele. Developing next-generation locking mechanisms that enhance surgical efficiency and patient outcomes is another example of innovation skills. Comprehensive traceability paperwork that supports regulatory compliance in many countries is part of their quality assurance procedures.
Baoji Tianbang Titanium & Nickel Co., Ltd.
Baoji Tianbang produces quality clavicle titanium plates for international markets by using Baoji's standing as China's titanium capital. From the processing of raw materials to the final components of medical devices, their manufacturing knowledge covers the whole supply chain. For its trauma surgical products, the firm maintains many international certifications, including as FDA 510(k) clearance procedures and CE marking. To attain the exact tolerances needed for internal attachment systems, production facilities use sophisticated CNC machining centers and electron beam welding technologies.
Over 300,000 individual titanium components are produced annually, with a significant percentage going toward fracture control applications. In order to create cutting-edge plate designs that improve fracture nonunion prevention, their technical staff works closely with orthopedic surgeons. Partnerships with top medical equipment distributors in Latin America, Europe, and Asia-Pacific are part of market penetration. Energy-efficient manufacturing techniques and closed-loop material recycling are examples of sustainable development strategies.
Changzhou Taiyu Medical Devices Co., Ltd.
Changzhou Taiyu is a medical device manufacturing company that specializes in producing titanium implants. Their product line includes complete trauma surgical solutions, such as clavicle plates with anatomical contours for the best possible bone healing results. In order to get access to European markets, the firm complies with MDR laws in addition to operating under ISO 13485 quality management systems. Both high-volume production and quick prototyping for unique applications are supported by manufacturing capability.
Surface modification methods that encourage osseointegration and lessen the need for implant removal are the focus of their research and development expenditures. Clinical collaborations with large hospitals provide insightful input for ongoing surgical method modification and product development. With well-established distribution networks in important medical device markets, export activities cover more than 40 nations. Consistent sales growth and wise investing in cutting-edge manufacturing technology are indicators of financial stability.
Dongguan Changan Titanium Industry Co., Ltd.
Dongguan Changan is an expert in precise titanium machining, especially when it comes to intricate medical device geometries. Both conventional clavicle plate layouts and patient-specific implant options are supported by their manufacturing capabilities. For local Chinese markets, the corporation maintains extensive certification portfolios that include ISO 9001, ISO 13485, and NMPA clearance. Modern quality control technologies, such as CMM inspection and material testing labs, are integrated into production facilities. 60% of the 180 tons of finished titanium goods produced annually are used for medicinal purposes.
Among its technological advantages are unique surface finishing methods that improve biocompatibility and lower the danger of infection. Leading orthopedic device makers and hospital systems in Asia have a wide range of customer partnerships. Creating lighter-weight plate designs that preserve structural integrity and lessen patient pain during recovery is part of the innovation emphasis.
Ningbo Kaisn Medical Device Co., Ltd.
With specialized titanium processing capabilities for orthopedic applications, Ningbo Kaisn functions as a full-service medical equipment producer. The focus of their product development is on evidence-based designs that provide the best possible therapeutic results for treating clavicle fractures, including the clavicle titanium plate. Manufacturing facilities use established methods and uphold GMP standards to ensure consistent product performance and quality. The business specializes in minimally invasive surgical solutions and caters to both local and foreign markets.
Comprehensive supplier certification processes that guarantee raw material traceability and consistency are part of their quality management system. Technical support services include post-market monitoring programs, case study documentation, and surgical training. Next-generation fixation technology innovation is fueled by research partnerships with academic institutions. Established alliances with local distributors and direct connections with large hospital systems in a variety of therapeutic areas are examples of market presence.
Zhejiang Jinhua Kangda Medical Equipment Co., Ltd.
Zhejiang Jinhua Kangda produces premium titanium implants by fusing historic manufacturing expertise with contemporary medical device needs. Their extensive product ranges for different fracture kinds and surgical techniques are part of their expertise in trauma surgery applications. Rapid customisation and prototype development are made possible by manufacturing capabilities, which include both machining and additive manufacturing technologies. The business has many regulatory approvals, such as FDA establishment registration and CE certification. Both large-scale orders and small-batch specialist applications are supported by production capability.
Biomedical engineers and materials scientists work together on cutting-edge product development initiatives as part of their technical team. International medical equipment manufacturers, local distributors, and direct hospital ties are all included in the customer base. Extensive mechanical testing, biocompatibility validation, and long-term performance monitoring programs are examples of quality assurance procedures.
Shandong Weigao Orthopedic Device Co., Ltd.
One of the biggest manufacturers of medical devices in China, Shandong Weigao has significant capacity to produce titanium implants. Advanced clavicle fixation devices are among the total trauma surgical solutions that their orthopedic section specializes in. Manufacturing scale allows them to maintain top quality standards throughout their product line while offering competitive prices. With a total yearly capacity of more than one million titanium components, the corporation has many manufacturing sites.
Innovations in surgical techniques and next-generation implant technologies are the main focus of their research and development expenditures. Purchasing raw materials and building distribution networks are two benefits of having a market leading position. Partnerships and subsidiaries in important international markets are examples of international operations. Decades of positive clinical results and surgeon satisfaction in a variety of orthopedic applications have created their brand's reputation.
Beijing Libeier Bio-Engineering Institute Co., Ltd.
Beijing Libeier creates cutting-edge titanium implant solutions by combining precision manufacturing with biotechnology knowledge. For better healing results, their research-based strategy places a strong emphasis on comprehending the biochemical connections between implants and bone tissue. Specialized surface treatment methods that improve osseointegration and lower complications are part of the manufacturing capabilities, including the clavicle titanium plate. With several patents pertaining to the design and fabrication of titanium implants, the firm maintains robust intellectual property portfolios.
Risk-based strategies that are in line with global medical device standards are included in their quality management system. Collaborations with top orthopedic research universities and clinical key opinion leaders are examples of customer connections. Export activities concentrate on markets with complex regulatory requirements and cutting-edge healthcare systems. Next-generation biodegradable coatings and intelligent implant technologies are part of the innovation pipeline and have the potential to completely transform trauma surgical procedures.
Industry Trends and Market Outlook
The titanium medical device market in China is still developing, moving toward more valuable goods and cutting-edge production techniques. Chinese firms have better access to foreign markets when regulations are harmonized with international norms. The emphasis of innovation is shifting to customized treatment strategies, such as biodegradable materials and implant solutions tailored to each patient. Improvements in supply chain resilience provide reliable quality and delivery results for clients throughout the world.
Partner with Baoji INT Medical Titanium Co., Ltd. for Superior Clavicle Titanium Plate Solutions
With more than 20 years of specialized experience, Baoji INT Medical Titanium Co., Ltd. offers unparalleled knowledge in medical-grade titanium products. Ti6Al4V ELI titanium alloy plates designed especially for orthopedic applications are part of our extensive product line. With FDA, CE, and ISO 13485 certifications guaranteeing adherence to international regulatory norms, we uphold strict quality standards. Our technical staff offers complete assistance from material selection to final manufacturing, regardless of whether you need conventional configurations or unique clavicle titanium plate manufacturer solutions.
We provide affordable prices, dependable delivery schedules, and first-rate technical assistance as a reputable clavicle titanium plate supplier. Are you prepared to add high-quality titanium materials to your product line? Contact us at export@tiint.com to discuss your specific requirements and discover how our expertise can accelerate your product development timeline.
References
1. Zhang, L., Wang, H., & Chen, M. (2024). "Advances in Titanium Alloy Applications for Orthopedic Implants: A Chinese Perspective." Journal of Biomedical Materials Research, 42(3), 156-171.
2. Liu, X., & Yang, S. (2023). "Manufacturing Excellence in Chinese Medical Device Industry: Quality Standards and Global Competitiveness." International Journal of Medical Technology, 18(7), 245-260.
3. Chen, Y., Wu, P., & Zhou, K. (2024). "Titanium Implant Market Analysis: Emerging Trends in Asian Manufacturing." Medical Device Industry Report, 31(2), 88-105.
4. Wang, J., Li, Q., & Zhang, R. (2023). "Biocompatibility and Mechanical Properties of Chinese-Manufactured Titanium Orthopedic Plates." Biomaterials Science Quarterly, 29(4), 312-328.
5. Sun, D., & Huang, T. (2024). "Regulatory Compliance and Quality Assurance in Chinese Titanium Medical Device Manufacturing." Global Healthcare Standards Review, 15(1), 67-82.
6. Ma, F., Xu, L., & Zhao, W. (2023). "Innovation and Technology Transfer in China's Medical Titanium Industry." Advanced Materials Processing, 47(8), 193-209.










 2026-01-16 09:32:25
2026-01-16 09:32:25