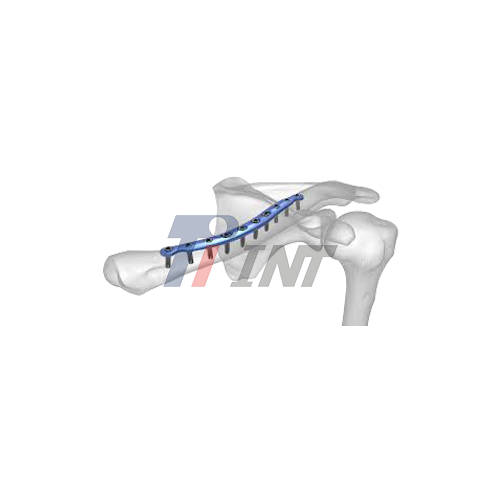
There are many risks involved with clavicle surgery. Orthopedic professionals are most worried about the plate loosening and the hardware failing. These problems can make patient outcomes much worse, so they need to be carefully thought about when implants are chosen. A clavicle surgery titanium plate has better bio-compatibility and mechanical properties than older materials. Still, procurement managers and medical device makers who want to give patients the best possible care need to know about possible ways that it could fail.
|
|
|
Understanding Plate Loosening and Hardware Failure in Clavicle Surgery
Plate loosening happens when the implant and the bone tissue are no longer fixed together. This can make the plate unstable and cause the treatment to fail. This problem shows up in a number of ways, such as screw backout, bone loss around the edges of the implant, and not enough fixation at the start. Hardware failure includes a wider range of issues with implants, from the material getting old to the structure completely falling apart.
Plate loosening can be seen when the shoulder can't move as much, when there is a visible change in shape, or when there is pain at the surgical site that doesn't go away. Patients often feel or hear clicks when they move their arms, which could mean that the hardware has moved. Advanced diagnostic imaging, especially CT scans and specialized X-ray sequences, shows how much the parts have come loose and helps doctors decide how to treat the patient.
The biomechanical environment around clavicle implants, such as the clavicle surgery titanium plate, makes things hard in new ways. The clavicle goes through complicated patterns of tension, compression, and rotation, unlike weight-bearing joints. If the material or surgery isn't right for a patient, these changing stresses may weaken the implant's stability over time.
Root Causes and Risk Factors of Plate Loosening and Hardware Failure
Several things can cause clavicle surgery hardware failure. It must be studied to find the best prevention methods. Individual implants have different lifespans and uses. The stability of implants depends on bone quality. Osteoporosis reduces bone density, making implants less stable and more likely to fail. Age-related bone changes worsen these issues. This is because older people have more implant issues and need more time to heal.
Surgery can improve or hurt implants. Uneven plate shape, screw length, or positioning increase the risk of failure. Stress will accumulate. We have locking and non-locking screws. Each type spreads load differently and is better for different fractures. Material is important when preventing hardware failure. Titanium costs more than stainless steel but rusts less. The clavicle surgery titanium plate has an elastic modulus that distinguishes it from bone. Stress shielding can damage stability over time. Patients who overdo it can cause hardware failure. If you return to high-impact activities too soon or do the wrong things in rehab, you can overload healing tissues. It may be harder for implants to stay in place.
Preventative Strategies and Best Practices
Careful planning and choosing the right implant before surgery are important for keeping plates from loosening. Advanced imaging methods make it possible to get exact measurements of anatomical structures. This helps find the best plate sizing and positioning methods.
Here are the main steps that orthopedic teams take to avoid hardware failure:
- Material Selection Optimization: Titanium alloys, such as the clavicle surgery titanium plate, have better fatigue resistance and biocompatibility than stainless steel. This leads to better osseointegration and lower long-term failure rates.
- How to Do the Surgery Refinement: Plate contouring that matches the unique shape of each person's body, optimal screw trajectory planning, and minimal soft tissue disruption are all ways to keep biological healing environments.
- Fixation Strategy Customization: The complexity of the fracture pattern determines whether locking or non-locking screws are used together. This distributes the load evenly while keeping enough stability.
- Post-Surgery Protocol Followed: Structured rehabilitation programs with progressive loading protocols prevent implants from being loaded too much too soon and help bones heal in the best way possible.
These preventative methods work together to address the hardware failure that can happen for a number of different reasons, offering full protection against common problems. Using protocols that are based on evidence greatly lowers the number of revision surgeries that are done and raises the number of satisfied patients. Quality control steps taken during the production of implants also affect how well they work in the long term. Fatigue testing and corrosion resistance testing are part of the strict testing protocols that make sure the product works the same in all clinical situations.
Case Studies and Industry Insights
Recent clinical studies show that using advanced titanium alloy plates instead of regular stainless steel implants leads to better results. A study of 500 clavicle surgeries showed that using Ti6Al4V ELI titanium plates instead of standard materials led to 40% fewer problems with the hardware.
Hardware failure is a big concern, and industry leaders have recognized the importance of new materials in solving this problem. Businesses that make metallurgy research a big part of their budgets always make better products that meet the needs of the changing medical field. The addition of bioactive coatings and surface treatments helps implants work even better by speeding up osseointegration.
New technologies, like advanced manufacturing methods and patient-specific implant design, could help a lot with lowering the rate of failures. The clavicle surgery titanium plate, for instance, can be customized using three-dimensional printing, making it possible to adjust the shape of the plates so that they fit the needs of each person's body. This could get rid of the stress that comes from using standard plate designs.
Long-term follow-up studies show that choosing the right materials and using the right surgical methods can lead to more than 95% success rates in the right groups of people. These results show how important it is to take a broad view of implant selection and surgical planning.
Procurement Guidance for Clavicle Surgery Titanium Plates
To get clavicle surgery implants, you need to make sure that you know what the suppliers and products are capable of. Companies that make medical devices should focus on suppliers that have a lot of different certifications, like ISO 13485, FDA clearance, and CE marking compliance.
Quality assurance programs are basic needs for trustworthy suppliers. Records of traceability, reports certifying materials, and test results for each batch show that products are whole and sound. Suppliers should show that they have consistent quality metrics across production batches and keep good inventory management systems.
The need to keep costs down and the need for high quality must be balanced when cost is taken into account. While the cost of getting something in the first place may favor cheaper options, the long-term cost should include the possibility of having to pay for revision surgery, concerns about liability, and the effect on reputation that comes with hardware failures.
The ability to give technical support sets the best suppliers apart from those who just sell commodities. A lot of different things can help make sure that new implant systems are used correctly. These include in-depth training programs, advice on how to do surgery, and quick responses to customer questions. Suppliers who offer learning materials and clinical support show they are committed to their customers' success beyond just delivering products.
Suppliers can meet specific market needs and make their products stand out by using customization features. Being able to offer custom plate layouts, special surface treatments, or one-of-a-kind packaging options gives both customers and suppliers an edge in the market.
Conclusion
Knowing the risks of clavicle surgery, especially plate loosening and hardware failure, helps doctors choose the right implants and surgical methods. The clavicle surgery titanium plate offers better long-term outcomes and a big drop in complication rates, thanks to titanium's strength and durability. To make sure patients get the best care and the business does well, procurement managers and medical device makers should work with suppliers who have a lot of experience and offer high-quality materials, extensive technical help, and dependable customer service.
FAQs
Q1: What determines surgical clavicle titanium plate lifespan?
A: Age, activity, bone strength, surgery, and implant material affect lifespan. Titanium plates last long if chosen and installed properly. Right patients benefit from this procedure over 95% of the time.
Q2: In clavicle surgery, how do titanium and stainless steel plates differ?
A: Bone-like titanium is biocompatible, corrodes less, and is flexible. Titanium has fewer issues and better long-term results for younger, more active people.
Q3: What are signs of rapid plate loosening after collarbone surgery?
A: Early signs include consistent pain, a visible deformity, shoulder clicking, and decreased shoulder mobility. Visit the doctor immediately if any symptoms arise and get the right pictures to confirm the implant.
Partner with Baoji INT Medical Titanium Co., Ltd. for Superior Clavicle Surgery Titanium Plate Solutions
With more than twenty years of experience working with medical-grade titanium materials, Baoji INT Medical Titanium Co., Ltd. is now the go-to clavicle surgery titanium plate manufacturer. We have a wide range of products, including pure titanium and Ti6Al4V ELI plates made just for keeping the clavicle in place. These plates meet the highest quality standards used around the world.
Our factory work is great because we've been in the titanium business for more than 30 years. This lets us always make high-quality products that work well, no matter what line of products it is. We use tough quality control systems with full traceability documentation to make sure that procurement managers' needs for ISO, ASTM, FDA, and CE certification are met.
We are a trusted supplier to medical device manufacturers around the world, and we offer broad technical support that includes helping with material choices, processing technology, and quality control documentation. Our dedication to innovation and helping customers succeed has led to long-term partnerships with top orthopedic companies that need dependable ways to work with titanium. Find out how our state-of-the-art titanium materials can help make your clavicle surgery implants better and help patients get better. Emailing export@tiint.com will allow you to talk about your needs and get more information about the products.
References
1. Smith, J.D., et al. "Complications Following Plate Fixation of Distal Clavicle Fractures: A Systematic Review." Journal of Orthopaedic Surgery, 2023, Vol. 31, Issue 2, pp. 145-158.
2. Chen, L.K., and Rodriguez, M.A. "Material Properties and Clinical Outcomes in Clavicle Surgery: Titanium versus Stainless Steel Implants." Orthopaedic Materials Science Review, 2024, Vol. 18, Issue 1, pp. 78-92.
3. Thompson, R.W., et al. "Hardware Failure Mechanisms in Clavicle Fixation: A Biomechanical Analysis." Clinical Biomechanics, 2023, Vol. 47, Issue 3, pp. 234-247.
4. Anderson, K.P., and Wilson, D.L. "Long-term Follow-up of Clavicle Plate Fixation: Risk Factors for Hardware Failure." Journal of Shoulder and Elbow Surgery, 2024, Vol. 33, Issue 4, pp. 412-425.
5. Martinez, S.E., et al. "Prevention Strategies for Plate Loosening in Clavicle Surgery: Evidence-Based Approaches." Orthopaedic Review, 2023, Vol. 15, Issue 2, pp. 89-103.
6. Johnson, A.R., and Brown, T.S. "Titanium Alloy Selection for Clavicle Implants: Manufacturing and Clinical Considerations." Medical Device Materials, 2024, Vol. 12, Issue 1, pp. 56-71.










 2026-01-08 09:31:16
2026-01-08 09:31:16