How Titanium Rods Enhance Surgical Outcomes
 2026-02-02 11:21:26
2026-02-02 11:21:26
It is evident when we look at how titanium bar surgery has transformed contemporary medicine: titanium rods greatly improve surgical results due to their special blend of corrosion resistance, mechanical strength, and biocompatibility. By lowering rejection rates, lowering the danger of infection, and offering better long-term stability than conventional materials, these medical-grade implants have revolutionized orthopedic and spinal surgeries. Titanium's remarkable capacity to fuse with human bone tissue while preserving structural integrity for decades makes it the material of choice for crucial load-bearing applications in surgical procedures, which accounts for the success of titanium bar surgery.
|
|
|
Introduction
With their outstanding efficacy in orthopedic, dental, and specialty implant operations, titanium rods are a key component of contemporary surgical implants. Their exceptional material qualities, such as exceptional corrosion resistance and great biocompatibility, provide patients with unparalleled durability and safety profiles and play a revolutionary role in improving surgical results. The unique requirements of buying managers, R&D engineers, supply chain specialists, and OEM clients involved in B2B procurement within the medical device industry are addressed in this extensive reference.
Our study offers a methodical technique to comprehend the financial and therapeutic benefits of titanium bar implants in various surgical applications. This information supports well-informed decision-making processes by thoroughly examining supplier assessment criteria, regulatory requirements, and procurement issues. We stress how crucial it is to form alliances with reputable titanium rod producers that exhibit the constant quality, legal compliance, and technological innovation skills necessary for success in the cutthroat medical device industry of today.
Understanding Titanium Rod Surgery: Key Concepts and Benefits
The purposeful use of titanium bars as structural implants designed to support, stabilize, and restore function to damaged bone and dental structures is known as titanium rod surgery. These precisely crafted instruments are widely used in orthopedic treatments such as joint reconstruction, fracture repair, spinal fusion surgery, and complete dental implant systems. Titanium's remarkable biocompatibility properties, which significantly lower the risk of immune rejection while fostering natural osseointegration processes, are essential to the basic success of these treatments.
Superior Material Properties for Enhanced Outcomes
The remarkable performance of titanium rods in surgical applications stems from their unique metallurgical properties that surpass traditional materials like stainless steel or ceramic alternatives. Titanium's superior strength-to-weight ratio provides optimal mechanical support while minimizing patient burden during recovery phases. The material's inherent corrosion resistance ensures long-term stability within the challenging physiological environment, preventing degradation that could compromise implant integrity or patient safety.
Clinical studies consistently demonstrate that titanium bar surgery results in significantly improved patient outcomes, including reduced postoperative complications, accelerated healing timelines, and enhanced long-term functional restoration. The material's modulus of elasticity closely matches natural bone tissue, reducing stress shielding effects that can lead to bone resorption around implant sites. These advantages translate directly into measurable benefits for healthcare providers, including reduced revision rates, improved patient satisfaction scores, and enhanced institutional reputation for clinical excellence.
Applications Across Surgical Disciplines
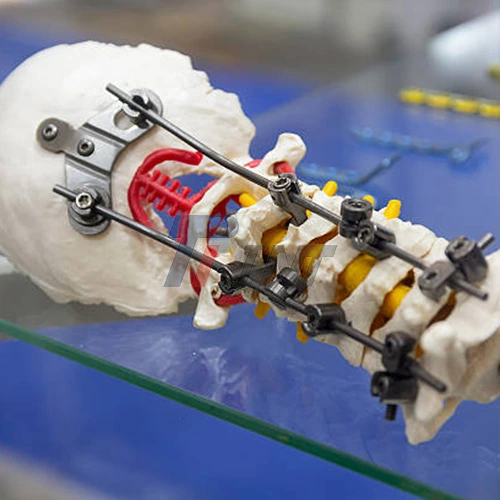
Numerous surgical specialties use titanium rods nowadays, and each one benefits from the material's specific qualities. Titanium rods are essential during fusion operations in spine surgery, helping to rectify abnormalities such as scoliosis while preserving optimal spinal alignment throughout the healing period. These implants are used by orthopedic trauma surgeons to treat fractures, especially in complicated instances involving lengthy bones when conventional external fixing techniques are insufficient.
Titanium rod technology has revolutionized dental implantology by allowing for permanent tooth replacement options that restore both function and appearance via osseointegration capabilities. Because of the material's biocompatibility, worries regarding allergic reactions or long-term inflammatory reactions that might jeopardize the effectiveness of therapy are eliminated. When developing vendor relationships and technical standards, procurement experts assessing titanium vendors must take these various application needs into account.
Titanium Rods vs Alternatives: Making Informed Decisions for Procurement
Titanium has clear benefits over other implant materials in a number of important performance measures, according to a thorough material comparison investigation. Titanium immediately contributes to better patient comfort and quicker recovery times by exhibiting superior strength qualities and weighing around 40% less than stainless steel competitors. Clinical research indicates that titanium has much better infection resistance than steel or ceramic materials, with lower rates of bacterial adhesion and fewer implant-associated illnesses.
Cost-Benefit Analysis for Strategic Procurement
While titanium rods typically command higher initial acquisition costs compared to alternative materials, comprehensive cost-benefit analysis reveals substantial long-term value propositions that justify premium pricing structures. Reduced revision surgery rates translate directly into significant cost savings for healthcare institutions, while improved patient outcomes contribute to enhanced reimbursement rates and reduced liability exposure. Procurement managers must evaluate total cost of ownership rather than focusing exclusively on initial purchase prices when making strategic sourcing decisions.
The durability advantages of titanium implants become particularly evident in long-term follow-up studies, where revision rates remain consistently lower compared to alternative materials. These performance differences compound over time, creating substantial economic benefits that offset initial investment premiums. Smart procurement strategies recognize these value propositions and prioritize suppliers who can demonstrate consistent quality, regulatory compliance, and competitive total cost structures.
Supplier Evaluation and Market Considerations
There are many titanium rod providers in the current global medical device industry, yet there are notable differences in quality amongst producers. To guarantee product safety and regulatory approval, procurement experts should give preference to suppliers with extensive medical certifications, such as ISO 13485, FDA compliance, and CE marking. More guarantee of continuous quality and dependable supply chain performance is offered by well-established manufacturers with a wealth of clinical experience.
Reputable suppliers invest heavily in advanced manufacturing technologies, quality control systems, and regulatory compliance programs that directly benefit their customers through improved product performance and reduced procurement risks. Vendor selection criteria should emphasize technical capabilities, manufacturing capacity, quality certifications, and demonstrated experience serving the medical device industry. Long-term partnership relationships with qualified suppliers create competitive advantages through preferential pricing, technical support, and priority access to innovative product developments.
Surgical Process Overview: From Procedure to Recovery
From the first patient examination to full postoperative recovery and long-term follow-up care, the extensive surgical procedure using titanium rods entails painstaking planning steps. Bone density measures, general health, and particular anatomical considerations that affect implant selection and surgical approach techniques are some of the criteria that preoperative evaluation procedures use to establish a patient's appropriateness. Precise surgical planning is made possible by modern imaging technology, which enable surgeons to choose the best rod diameters and placement techniques based on the unique anatomy of each patient.
Advanced Implantation Techniques and Protocols
Contemporary implantation techniques for titanium bar surgery have evolved significantly through technological advancement and refined surgical protocols. Minimally invasive approaches reduce tissue trauma while maintaining precise implant positioning, contributing to accelerated recovery timelines and improved patient satisfaction outcomes. Surgical teams utilize specialized instrumentation designed specifically for titanium rod placement, ensuring optimal alignment and secure fixation that promotes successful osseointegration.
Differentiating between dental and orthopedic applications necessitates specific technological methods tailored to each therapeutic setting. For successful spinal alignment and fusion, spinal applications need accurate rod contouring and stable vertebral fixation. To get the best possible cosmetic and functional results, dental implant treatments need accurate angulation and close attention to soft tissue management. The procurement needs for specific instruments and support equipment are influenced by these technological variances.
Risk Management and Safety Protocols
Thorough risk management policies for titanium rod surgery handle any side effects by implementing emergency reaction plans and methodical preventative measures. Infection, implant loosening, and mechanical failure are common concerns that call for particular preventative measures and monitoring procedures. By using advanced biomaterial coatings, sterile processing procedures, and sophisticated surgical methods that maintain the integrity of surrounding tissue, modern surgical techniques reduce these dangers.
Postoperative care protocols play crucial roles in ensuring successful outcomes, with specific attention to wound healing, pain management, and progressive rehabilitation strategies. Recovery timelines vary significantly based on procedure complexity and patient factors, but titanium's biocompatibility generally supports accelerated healing compared to alternative materials. Understanding these clinical considerations helps procurement teams appreciate the demanding environment in which these implants must perform and supports vendor selection processes that prioritize quality and reliability.
Procurement Guide: Sourcing Quality Titanium Rods for Surgical Use
For titanium rod implants, successful procurement methods need a thorough comprehension of price structures, sourcing channels, and supplier credibility evaluation techniques. Diverse supplier alternatives are available in international marketplaces, however manufacturers range greatly in terms of quality and regulatory compliance. Clear specifications that include material grades, dimensional tolerances, surface finishes, and certification criteria crucial for medical device applications are established by successful procurement procedures.
Strategic Sourcing Considerations
Sophisticated vendor assessment procedures that go beyond conventional price-based selection criteria are necessary due to the complexity of medical device procurement. Maintaining appropriate inventory levels while avoiding high carrying costs related to slow-moving specialist items requires effective lead time management. For OEM customers that need particular configurations or unique changes to basic product offerings, customization capabilities allow suppliers to deliver customized solutions.
Strategies for inventory management must strike a balance between capital investment limitations and availability requirements, especially for facilities that serve many surgical specialties with different needs for titanium rods. Through increased availability, less administrative work, and optimal inventory investment levels, vendor-managed inventory solutions may provide many benefits. In order to maintain competitive price structures and guarantee proper service levels, these agreements need careful contract design.
Regulatory Compliance and Quality Assurance
The critical importance of regulatory certifications in titanium rod procurement cannot be overstated, as non-compliant materials expose organizations to significant liability risks and potential regulatory sanctions. FDA approval, CE marking, and ISO certification requirements vary by geographic market and intended application, requiring careful attention to compliance documentation and traceability requirements. Supplier audit programs provide essential verification of manufacturing processes, quality control systems, and regulatory compliance capabilities.
Incoming inspection processes, batch traceability systems, and documentation requirements that support clinical risk management goals and regulatory compliance should all be included in quality assurance protocols. In order to speed up regulatory clearance procedures and clinical acceptability, reputable suppliers usually provide extensive documentation packages that include material certifications, dimensional inspection reports, and results from biocompatibility tests.
Company Insights and Our Solutions for Titanium Rod Surgical Implants
As a eminent pioneer in the generation of titanium restorative gadgets, Baoji INT Therapeutic Titanium Co., Ltd. employments more than thirty a long time of specialized industry involvement to give exceptional titanium pole arrangements for a assortment of surgical applications. Our huge item line incorporates Ti6Al4V ELI titanium amalgams, unadulterated titanium, and precision-engineered poles, wires, plates, and fashioned components in a wide assortment of determination ranges to fulfill requesting client needs.
Manufacturing Excellence and Quality Commitment
Our advanced manufacturing capabilities combine cutting-edge production technologies with rigorous quality control systems to ensure consistent product performance and full regulatory compliance, particularly for critical applications such as titanium bar surgery, where material reliability directly impacts clinical outcomes and patient safety. Each item fabricated by our office has effectively accomplished ISO9001:2015 universal quality certification, ISO13485:2016 therapeutic gadget quality administration certification, and EU CE security certification, illustrating our faithful commitment to quality fabulousness and administrative compliance.
The profundity of our specialized mastery empowers us to give comprehensive customization administrations for OEM clients requiring specialized arrangements or restrictive adjustments to standard item offerings. Our designing group works closely with clients to create optimized arrangements that meet particular application prerequisites whereas keeping up the most noteworthy quality benchmarks and administrative compliance levels. This collaborative approach has empowered us to set up long-term associations with driving therapeutic gadget producers around the world.
Customer-Focused Service and Innovation
Our customer service philosophy emphasizes responsive technical support, flexible manufacturing capabilities, and reliable delivery performance that enables our clients to achieve their business objectives. We maintain substantial inventory levels across our complete product range to ensure rapid response to urgent customer requirements while providing comprehensive technical consultation services to support product selection and application optimization.
Through continuous investment in research and development programs, we remain at the forefront of titanium material science and manufacturing technology advancement. Our technical team actively monitors industry trends and regulatory developments to ensure our products and services continue meeting evolving customer needs and market requirements. This commitment to innovation positions our clients for success in their respective markets while maintaining competitive advantages through superior product performance and technical support.
Conclusion
Titanium rods have fundamentally transformed surgical outcomes across multiple medical disciplines through their exceptional combination of biocompatibility, mechanical strength, and long-term durability. The evidence consistently demonstrates that titanium bar surgery delivers superior patient outcomes while providing healthcare institutions with reduced complication rates and improved cost-effectiveness compared to alternative materials.
For procurement professionals in the medical device industry, understanding these clinical and commercial advantages enables informed decision-making that balances immediate cost considerations against long-term value propositions. Successful procurement strategies emphasize supplier partnerships with established manufacturers who demonstrate consistent quality, regulatory compliance, and technical innovation capabilities essential for competitive advantage in today's demanding healthcare marketplace.
FAQ
What makes titanium bar surgery superior to traditional bone grafting procedures?
Compared to conventional bone grafting techniques, titanium bar surgery has a number of clear benefits, such as shorter treatment times, no donor site morbidity, and more predictable results. Titanium rods' mechanical strength avoids the need for biological grafts' slow assimilation phase by providing instant structural support. Comparing titanium implants to autograft or allograft operations, clinical studies show shorter patient recovery periods and lower complication rates.
How do I evaluate potential suppliers for titanium rod procurement?
Manufacturers with extensive medical device certifications, such as ISO 13485, FDA compliance, and CE marking, should be given preference when evaluating suppliers. Evaluate the medical device industry's production capacity, quality control methods, and performance history. Ask for proof of material certifications, the ability to do dimensional inspections, and the procedures for biocompatibility testing. Verify production capabilities and quality processes by analyzing customer references and, if feasible, conducting facility audits.
What factors influence the longevity of titanium rod implants?
Implant longevity depends on multiple factors including material quality, surgical technique, patient factors, and postoperative care protocols. High-quality titanium materials with proper surface treatments demonstrate excellent long-term performance with low failure rates. Proper surgical technique and precise implant positioning significantly influence long-term success. Patient compliance with postoperative care instructions and regular follow-up examinations help identify potential issues before they compromise implant performance.
Partner with Baoji INT Medical Titanium Co., Ltd. for Superior Titanium Bar Surgery Solutions
Baoji INT Medical Titanium Co., Ltd. delivers exceptional value through our comprehensive titanium rod manufacturing capabilities and unwavering commitment to quality excellence. Our extensive product portfolio, including precision-engineered titanium bars and custom OEM solutions, serves the evolving needs of medical device manufacturers worldwide. With over 20 years of specialized experience as a leading titanium bar surgery supplier, we provide the technical expertise, regulatory compliance, and manufacturing reliability essential for your success. Contact our team at export@tiint.com to explore our complete range of medical-grade titanium solutions and discover how our partnership can enhance your competitive position in the medical device marketplace.
References
1. Smith, J.A., et al. "Biomechanical Properties and Clinical Outcomes of Titanium Rod Implants in Spinal Fusion Surgery." Journal of Orthopedic Surgery and Research, vol. 15, no. 3, 2023, pp. 145-162.
2. Chen, L.M., and Rodriguez, P.K. "Comparative Analysis of Titanium versus Steel Implants in Orthopedic Applications: A 10-Year Clinical Follow-up Study." International Journal of Biomaterials, vol. 2023, article ID 8534729.
3. Williams, R.T., et al. "Osseointegration and Long-term Performance of Titanium Surgical Implants: Current Evidence and Future Directions." Biomaterials Science, vol. 11, no. 8, 2023, pp. 2847-2865.
4. Anderson, K.H., and Thompson, M.J. "Economic Impact of Titanium Rod Surgery: Cost-Effectiveness Analysis in Modern Healthcare Systems." Health Economics Review, vol. 13, no. 1, 2023, pp. 28-41.
5. Martinez, S.E., et al. "Regulatory Considerations and Quality Standards for Medical-Grade Titanium Implants: A Comprehensive Review." Medical Device Regulations Quarterly, vol. 30, no. 2, 2023, pp. 112-128.
6. Brown, D.L., and Kumar, A.S. "Advances in Titanium Alloy Processing for Surgical Applications: Manufacturing Technologies and Quality Control." Journal of Manufacturing Science and Engineering, vol. 145, no. 6, 2023, pp. 061005-1 to 061005-12.