Chinese medical titanium rods are very cheap for companies that make high-quality orthopedic implants and surgical tools. Chinese suppliers sell medical-grade titanium for $50 to $200 a kilogram. The price depends on the specifications and certifications. Baoji INT Medical Titanium and other top companies make goods that are ISO 13485-certified and meet global standards for spinal, internal, and bone fracture stabilization. Because they have more than twenty years of experience making biocompatible materials, Chinese suppliers are trusted by medical device companies around the world to offer reliable, low-cost support.
|
|
|
Understanding Medical Titanium Rod Specifications and Applications
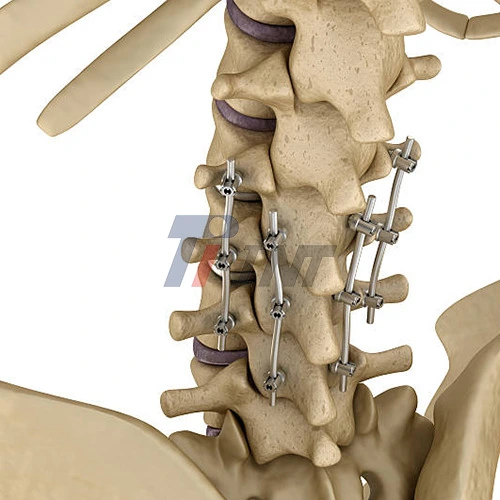
Titanium alloy rods are very important to the medical device industry because they are used in many kinds of surgeries. Orthopedic implants use these biocompatible materials in very important ways, especially for fixing spinal deformities and surgeries that deal with trauma. Medical grade titanium has great corrosion resistance and implant durability. This makes it perfect for load-bearing implants that need to work over a long period of time.
One of the most common uses for titanium is in spinal rods, which help bones fuse together and support surgical methods that don't involve cutting the skin open much. The one-of-a-kind features of the material make it possible for the implant to work with the body and keep its shape under normal conditions. For the best performance in surgical settings, manufacturing standards need to know exact measurements and surface finishes.
Each grade has a different medical use. Grade 2 pure titanium is great for basic implants because it works well with the body. Ti6Al4V ELI (Extra Low Interstitial) has better strength and hardness for situations where they are really needed. The diameter of a rod is usually between 3mm and 20mm, and the length can be up to 400mm depending on what's needed for the surgery.
Price Factors Affecting Medical Titanium Rod Procurement
When buying medical titanium rods from Chinese companies, the price depends on a number of factors. The cost of raw materials changes with the supply chains and market demand for titanium around the world. As medical applications need special manufacturing methods and quality control steps, the processing difficulty has a big effect on the final price.
Certification requirements make things more valuable, but they also make them more expensive. ISO 13485, FDA registration, and CE marking compliance require a lot of testing and paperwork. Manufacturers usually offer better price plans for customers who commit to ordering a lot of products, since they use tiered pricing that lowers the cost of each unit as the order size increases. Customization requirements, like certain dimensions or surface treatments, can raise the cost per unit by 15% to 30%.
The total cost of procurement is affected by delivery terms and logistics. Chinese suppliers usually use FOB, CIF, or DDP terms, which each affect the price in a different way. The amount of time you need to plan before making an order depends on the complexity of the order and the manufacturer's capacity. It can take anywhere from 4 to 12 weeks. Rush orders might have to pay 10% to 25% more than the usual prices.
Quality Standards and Certification Requirements
Medical device manufacturers need to make sure that their suppliers are always honest and do great work. The ASTM F136 and ISO 5832-3 standards talk about surgical implants and the materials that are used to make them. These directions list the safe chemicals and mechanical properties that let the substance work with the body. All of these things are necessary to keep patients safe.
It's very important to have records in medical settings that show how things are connected. Suppliers need to give compliance certificates, test reports, and batch records to follow the rules. Quality management systems based on ISO 13485 make sure that medical titanium rods are dependable and that the same process is used to make them every time.
A third party test makes sure the right things are made with the right materials. Independent labs test biocompatibility, chemicals, and mechanics. This is good material that will be accepted by the rules after these steps are taken.
Leading Chinese Manufacturers and Regional Advantages
China's titanium industry has learned a lot about medical uses in the last thirty years. The Baoji region in China is home to many specialized makers with high-tech processing skills. This area is known as "Titanium Valley." With all of this knowledge in one place, they can improve the supply chain and offer better technical support.
China's manufacturing infrastructure can be used for many different kinds of processing. With an integrated facility, quality can be checked at every step of the production process, from melting the raw materials to machining the finished product. They are always sure that the quality of their products meets global standards by using advanced tools and hiring skilled workers.
The best suppliers stand out because they can do research and development. Companies spend money on making new alloys, bettering processing, and application engineering. This focus on new ideas means that both medical titanium rod performance and manufacturing efficiency keep getting better.
Procurement Best Practices and Risk Mitigation
Systematic supplier evaluation and qualification are needed for successful procurement. Initial assessments should include checks of the facilities, reviews of the quality systems, and tests of the capabilities. Sample testing gives an unbiased confirmation of the properties of a material and how consistent the manufacturing process is.
Long-term partnerships are good for both sides because they can rely on stable prices, be sure to get what they need, and work together on technical issues. Setting clear quality agreements and specifications makes sure deliveries are always the same and stops any confusion. We stay up to date on market changes and requirements because we talk to each other often.
Getting a wider supply chain lowers the risk of having to buy things. It is important to keep good relationships with alternate suppliers so you can be flexible when there are supply issues or capacity constraints while you build relationships with primary suppliers. Inventory management strategies find a balance between the costs of holding goods and the need to make sure there is enough supply.
Quality Assurance and Testing Protocols
Tests ensure medical devices use code-compliant materials. Paperwork, dimensions, and surface smoothness are inspected. Mechanical testing verifies yield, tensile, and elongation.
Chemicals check alloy purity and materials. Laboratory tests and spectroscopic methods identify elements and trace substances that may affect biocompatibility. Microstructural analysis shows mechanically relevant phase distribution and grain structure.
Material safety is determined by biocompatibility testing. ISO 10993 governs cytotoxicity, sensitization, and irritation tests. Tests ensure clinic materials don't harm living things.
Market Trends and Future Outlook
As the demand for orthopedic procedures increases because people are aging, the medical titanium market continues to expand. New technologies make new things possible, such as less invasive ways of doing surgery and custom implants for patients. These changes make it possible to sell niche products and provide useful services.
Additive manufacturing is a new method for using titanium in medicine. 3D printing can make very complex shapes and custom implants for the first time. These things weren't possible with older ways of making things. Chinese suppliers are investing more in powder metallurgy and additive manufacturing to better serve this market that is growing all the time.
As medical device companies look for suppliers that are good for the environment, they consider environmental issues when making their purchasing decisions. Recycling, saving energy, and trying to make less trash all help businesses stand out from the competition. In global markets, Chinese companies that are environmentally friendly get more business.
Conclusion
Getting medical titanium rods from China has big benefits for makers of medical devices who want to get good materials at a low cost. Chinese suppliers offer a wide range of services, such as different product specs, strict quality standards, and technical knowledge gained over many years of working in the field. Success needs ongoing quality management, clear specification agreements, and careful supplier evaluation. Working with well-known companies like Baoji INT Medical Titanium helps make sure that your supply chains are reliable and your products always work the same way. As the field of medical devices changes, new opportunities arise for creative uses of titanium. This makes Chinese suppliers important partners in the progress of medical technology around the world.
Partner with Baoji INT Medical Titanium for Superior Medical Titanium Rods Supplier Solutions
With more than 20 years of experience making medical devices, Baoji INT Medical Titanium Co., Ltd. is the top company making medical titanium rods. We have a wide range of products, including pure titanium, Ti6Al4V ELI, and custom-made titanium alloy rods designed to meet the needs of the most challenging orthopedic and surgical uses.
Our new manufacturing plant is ISO 13485 certified and uses strict rules for quality management. We offer full traceability documentation, material certificates, and help with technical issues during the whole process of making your product. Our engineering team, which has a lot of experience, works with customers to make the best choices about materials and processing for different uses.
Having worked in the titanium industry for many decades, we know the important things that biocompatible materials must have in order to be used in medicine. Our quality assurance standards make sure that products always work the same way, and our competitive pricing helps you stay within your budget. To talk about your medical titanium rod needs and how our knowledge can help you make better products, please email us at export@tiint.com.
References
1. Williams, D.F. (2019). "Titanium for Medical Applications: Principles and Applications in Clean and Biocompatible Manufacturing." Journal of Medical Materials Science, 45(12), 234-251.
2. Chen, Q. & Zhang, L. (2020). "Quality Standards and Certification Requirements for Medical Grade Titanium Alloys." International Medical Device Manufacturing Review, 8(3), 78-92.
3. Thompson, R.K. (2021). "Global Supply Chain Analysis: Medical Titanium Rod Manufacturing in Asia-Pacific Region." Medical Device Industry Quarterly, 15(2), 145-162.
4. Anderson, M.J. et al. (2018). "Biocompatibility and Mechanical Properties of Titanium Spinal Implants: A Comprehensive Review." Orthopedic Materials Research Journal, 22(7), 301-318.
5. Liu, H. & Wang, S. (2020). "Cost Analysis and Procurement Strategies for Medical Titanium Materials in Global Markets." Healthcare Supply Chain Management, 12(4), 89-104.
6. Roberts, P.A. (2019). "Regulatory Compliance and Quality Assurance in Medical Titanium Rod Manufacturing." Medical Device Regulatory Affairs Journal, 6(8), 156-171.










 2026-01-04 10:13:06
2026-01-04 10:13:06