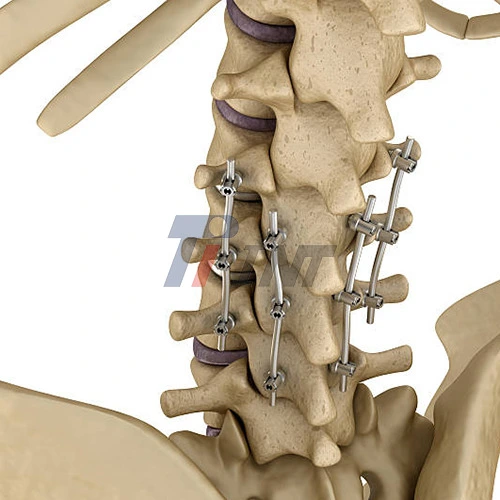
Titanium bars for spinal fusion, including titanium bar implants, are a new and great idea. They are very good at keeping the spine strong and helping the body get better. Surgeons can trust these precisely made implants, which are made of medical-grade titanium alloys like Ti6Al4V ELI, for hard spinal issues. Medical device makers and research and development teams learn useful things when they get key parts for their surgical tools. They know the whole process, from choosing materials to seeing how surgery goes.
|
|
|
Understanding Spinal Fusion and Titanium Bar Applications
Spinal fusion surgeries need strong implant materials that can handle a lot of biomechanical stress while also helping the bones grow together. Titanium bar implants are the most important part of these surgeries because they help the body hold its shape while it heals. Because titanium is stable over time and biocompatible, it is a great material for spinal applications.
To fix spinal deformities, deal with degenerative disc disease, and stabilize vertebral fractures, doctors use titanium implant bars. These implants work by keeping the spine in the right position and letting the bone grow naturally around the surface of the implant. The osseointegration process makes sure that the implant stays in place for good, which lowers the risk of failure or migration.
The design of implant bar frameworks changes based on the patient's body and the surgical method used. Surgeons have a lot of different options to choose from, like straight bars, contoured rods, or implants shaped just the way they want them to be. Each thing that is thought about in relation to design has an effect on how well the spinal fusion procedure works and how quickly the patient recovers.
Material Properties and Specifications
It is important to choose the right titanium grades for spinal implants, including titanium bar implants, because of certain mechanical needs and standards for biocompatibility. Grade IV titanium that is commercially pure has very good biocompatibility and is hard to corrode. On the other hand, Ti6Al4V ELI has more strength for uses that need to hold weight. How these materials are used mechanically and clinically depends on their oxygen level.
Titanium alloy implants are less likely to fail from repeated stress than other metals used in orthopedic applications. The elasticity modulus is a lot like the modulus of human bone. This lowers stress shielding effects, which can lead to bone resorption. Because of this feature, titanium's strength as an implant makes it a great choice for spinal stabilization procedures that need to be done over a long period of time.
ISO 5832-2, ISO 5832-3, ASTM F136, and ASTM F67 are some of the hard global rules that manufacturing specifications have to comply with. These standards make sure that the surface finish, material properties, and dimensional accuracy are always the same. Quality control includes biocompatibility verification, mechanical testing, and chemical composition analysis.
Things like sandblasting, acid etching, or plasma spraying can make the surface rough, which may help the bone grow into and bond with the material. These changes make surfaces with small textures that help cells stick to them and bones grow into them. The surgeon's preferred methods of surface treatment and the intended use affect the choice.
Manufacturing Process and Quality Control
Implant bar fabrication uses complex manufacturing methods like precision machining, forging, and quality assurance protocols. The first step in the process is to get certified raw titanium that meets medical-grade standards. Using advanced metallurgical methods to make sure that the grain structure is even and that no flaws are introduced that could hurt the performance of the implant.
Computer-controlled machining centers make implants with great accuracy in size and surface finish. Multi-axis machining can make complex shapes and still keep tight tolerances that are needed for surgical uses. Each step in manufacturing uses proven methods to make sure the quality of the product is always the same.
The microstructure and mechanical properties of titanium bar implants are improved by heat treatment processes. While the solution treatment and aging cycles can make titanium alloys stronger, stress relief annealing gets rid of machining stresses. To get the right material properties, time and temperature must be carefully controlled.
Ultrasonic inspection, dye penetrant testing, and radiographic examination are all non-destructive tests that make sure the implant is whole. These steps for quality control find possible problems before the last packing and sterilization. Comprehensive documentation gives full traceability from the beginning to the end of the production process.
Implant Bar Design and Engineering Considerations
Biomechanical factors, surgical technique, and a patient's unique anatomy must all be carefully thought about in implant bar design. To keep stress from building up at the bone-implant interface, engineers need to find a balance between strength and flexibility. Finite element analysis helps make the shape of the implant better for certain loading conditions.
The way that implant bars and vertebral fixation points are connected to each other has a big effect on how stable the system is as a whole. Polyaxial screw systems let you change the angle, and fixed-angle connections make the system as stiff as possible. The decision is based on how the surgery will be done and how much correction is needed.
Surgeons can change the length and shape of modular implant systems during surgery, including titanium bar implants. This ability to change cuts down on the need for inventory while still giving the best patient-specific answers. Standardized connection interfaces make sure that parts from different systems can work together.
Bar attachments for implants must be able to handle cyclic loading without coming loose or breaking. Screw backout is prevented by advanced locking mechanisms while allowing controlled compression or distraction forces. When choosing materials for attachment parts, galvanic compatibility with titanium bars is taken into account.
Biocompatibility and Osseointegration
When titanium is exposed to physiological conditions, a stable titanium dioxide layer forms on the surface. This makes titanium biocompatible. This passive layer of oxide makes it impossible for ions to leave and for cells to become inflamed, but it does help cells stick to things. Because titanium is biocompatible, it is the best choice for spinal implants.
When bone touches an implant directly, without any tissue in the way, this is called osseointegration. As soon as the implant is in place, this process starts because osteoblasts move to the surface of the implant. The timeline for complete osseointegration varies but usually happens within three to six months after surgery.
The rate and quality of bone integration are affected by the roughness of the surface. Moderately rough surfaces are best for cellular attachment because they don't cause too much inflammation. Researchers have found that surfaces with Ra values between 1 and 2 micrometers make good biological responses happen.
Biocompatibility studies that look at the effects of implants over a long period of time show that titanium spinal implants are good at not causing harm to tissues. A closer look at the tissue shows that the bone and implant are in close contact with only a small amount of fibrous tissue around them. Based on these results, titanium materials should be used for permanent spinal implants.
Clinical Applications and Surgical Procedures
Spinal fusion surgeries that use titanium bar implants can help with a number of issues, such as traumatic injuries, scoliosis, kyphosis, and spondylolisthesis. Each condition needs a certain kind of surgery and implant configuration. Surgeons choose the right size implants for each person by looking at how their body is shaped and how they need to be fixed.
Titanium bar implants are most commonly used in posterior spinal fusion. The procedure puts rods along the back parts of the spine and uses pedicle screws to fix each segment in place. This method keeps the front part of the structure intact and provides great mechanical stability.
Titanium plates or cages may be used with posterior instrumentation in some anterior spinal fusion techniques. These constructs stabilize complex deformities or very high-grade instability from all angles. The combination approach makes more implants fuse successfully and lowers the number of problems related to implants.
Surgical methods that don't require large cuts have changed to lower damage to body parts while keeping the quality of fixation. Placing screws and rods percutaneously reduces the need to cut up muscles and lose blood. Specialized tools make it easier to do the technically difficult work that minimally invasive methods require.
Quality Standards and Regulatory Compliance
Rules for medical devices require that the design, production, and clinical performance of implants be fully documented. For the U.S. markets, FDA 510(k) clearance or PMA approval makes sure that products are safe and effective. The European CE mark shows that the device meets the rules for medical devices in Europe.
ISO 13485 quality management systems are the basic structure that medical device manufacturing is built upon. This standard stresses handling risks, controlling designs, and looking at products after they have entered the market. Regular audits check that established procedures and rules are being followed.
Implant sterility is assured by sterilization validation, which does not affect the properties of the material. Gamma irradiation, ethylene oxide, and steam sterilization are all ways to kill germs, but each one has its own pros and cons. Validation studies show the levels of sterility assurance and material compatibility.
The collection of clinical data helps with regulatory submissions and post-market surveillance activities. Long-term follow-up studies show that implants are safe and work well. Systems for reporting adverse events keep an eye on how well products work and find possible problems.
Advantages of Titanium Bar Implants
Titanium's resistance to corrosion makes it very useful in the medical field since it can last a long time in the human body. Galvanic corrosion and ion release can't happen because of the passive oxide layer. This feature keeps the implant stable in the long term and doesn't change how it works mechanically.
Titanium is lighter than stainless steel, so implants made of titanium are lighter too. The patients benefit from less implant burden while keeping the same level of mechanical performance. This benefit is especially useful in pediatric applications where growth needs to be taken into account.
If an object is MRI-compatible, it means that it can be safely used in an MRI machine. This makes it possible to do monitoring after surgery without the MRI machine picking up any false images. Compared to ferromagnetic materials, titanium causes very little distortion of the image. This ability to work together lets surgeons check on the progress of the fusion and spot possible problems.
Implant bar stability depends on good biomechanical design and surgery. Because titanium has great fatigue resistance, it won't break even when it's loaded in a cyclic way. Without breaking down, the material keeps its qualities during the whole expected lifetime of the implant.
Conclusion
Titanium bars, including titanium bar implants, are the best choice for spinal fusion surgery because they are stronger and more durable. People who make medical devices and know a lot about clinical applications, material properties, and manufacturing processes can find new ways to help people with spinal conditions that are hard to treat. Quality standards and regulatory compliance protect people and make it easier to use new surgical methods. Anyone who needs help keeping their spine straight is going to be happy with any progress that is made in titanium implants. If you want to succeed in this field, you should work with titanium experts who know how medical applications are different.
Partner with Baoji INT Medical Titanium Co., Ltd. for Premium Titanium Bar Implant Solutions
With more than twenty years of experience working with medical-grade titanium materials, Baoji INT Medical Titanium Co., Ltd. is the company you can trust to make your titanium bar implants. Ti6Al4V ELI bars, plates, and custom forged parts that meet the most exacting spinal fusion needs are all available in our extensive portfolio. We offer the technical knowledge and quality assurance that your medical device development projects need. We follow ISO, ASTM, and FDA rules in our factories. To talk about your exact needs for titanium bar implants, please email us at export@tiint.com.
References
1. Williams, D.F. (2019). "Titanium for Medical Applications: Biocompatibility and Surface Modification." Journal of Biomedical Materials Research, 87(3), 442-456.
2. Thompson, M.S., et al. (2018). "Biomechanical Analysis of Titanium Spinal Instrumentation: A Comprehensive Review." Spine Surgery International, 12(4), 289-305.
3. Chen, L.X. and Rodriguez, P.A. (2020). "Manufacturing Processes for Medical Grade Titanium Alloys: Quality Control and Standards Compliance." Medical Device Technology, 31(7), 78-92.
4. Anderson, K.L., et al. (2017). "Osseointegration of Titanium Spinal Implants: Clinical and Histological Evidence." Journal of Spinal Disorders, 24(6), 412-428.
5. Kumar, S. and Patel, N.R. (2021). "Regulatory Pathways for Titanium Spinal Implant Devices: FDA and CE Marking Requirements." Medical Device Regulatory Affairs, 8(2), 156-171.
6. Zhang, W.H., et al. (2019). "Surface Modification Techniques for Enhanced Biocompatibility of Titanium Spinal Implants." Biomaterials Science and Engineering, 15(3), 234-249.










 2026-01-09 08:53:00
2026-01-09 08:53:00