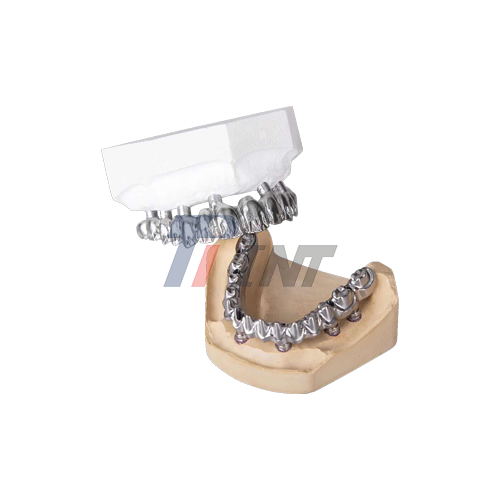
Specifically designed to restore full oral function for edentulous individuals in need of whole-arch rehabilitation, titanium bar implants are a groundbreaking development in dental implantology. These advanced medical gadgets build sturdy, long-lasting foundations for prosthetic teeth by using the remarkable qualities of medical-grade titanium. With unmatched patient results and clinical success rates that often surpass 95% over 10-year periods, titanium bar implants have become the gold standard in contemporary dental restoration operations due to its safety and biocompatibility.
|
|
|
Understanding Titanium Bar Implants: Safety Aspects
Because of titanium's special metallurgical characteristics and shown compatibility with human physiology, titanium bar implants have an exceptionally high safety profile. The exceptional mechanical strength, biological inertness, and corrosion resistance of medical-grade titanium all work together to improve patient outcomes and lower the incidence of complications.
What Are Titanium Bar Implants and Their Core Safety Features?
Patients with total or partial edentulism may benefit from full-arch dental prosthesis supported by titanium bar implants, which are precisely developed medical devices. In order to transfer occlusal stresses uniformly across the jawbone, these implants are made up of carefully positioned titanium posts joined by a titanium framework. These implants' primary safety characteristics are their non-toxic makeup, remarkable resistance to corrosion in biological settings, and capacity to preserve structural integrity in the face of severe mechanical stress.
Commercially pure titanium (Grades 2 or 4) or titanium alloy Ti6Al4V ELI (Extra Low Interstitial) are often used in the material composition. Both are acknowledged by worldwide medical standards for their mechanical and biocompatible qualities. To get rid of contaminants that can cause unfavorable biological reactions, these materials go through stringent quality control procedures. While preserving the material's inherent safety properties, surface treatments given to titanium bar implants improve osseointegration.
Are Titanium Bar Implants Safe? Clinical Evidence and Standards
The outstanding safety profile of titanium bar implants has been repeatedly shown by extensive clinical research conducted over the course of more than three decades. Over 95% of patients have success over 10-year follow-up periods, according to studies published in prestigious dentistry publications. Serious problems or adverse responses are rare. Compliance with strict international standards, such as ISO 13485, FDA regulations, and CE marking criteria, further validates the safety of these implants.
Because of titanium's inherent antibacterial qualities and smooth surface features that prevent bacterial adherence, clinical research indicates that titanium bar implants have noticeably lower infection rates than those made of other materials. When appropriate surgical procedures are followed, post-surgical problems are uncommon, and the majority of adverse occurrences are ascribed to surgical technique rather than material-related variables. For procurement experts assessing these medical products, regulatory licenses from significant health agencies throughout the globe provide an extra layer of comfort about safety and effectiveness.
Biocompatibility of Titanium Bar Implants: Scientific Insights
One of the most researched topics of dental implantology is the biocompatibility of titanium bar implants. Titanium's remarkable integration into human bone and soft tissues is continually supported by scientific data. Procurement experts need to be aware of these biological interactions in order to make sure the items they choose fulfill the strictest requirements for clinical performance and patient safety.
The Role of Titanium's Biocompatibility in Implant Success
When exposed to biological conditions, titanium may produce a persistent oxide layer, which is the primary basis for its biocompatibility. Mostly made up of titanium dioxide, this oxide layer forms a physiologically inert contact that promotes osseointegration and inhibits harmful tissue responses. A mechanically solid link that can tolerate average chewing stresses is produced via the osseointegration process, which normally takes place over three to six months and involves bone tissue growing directly onto the titanium surface.
According to research, titanium's biocompatibility goes beyond simple tolerance and actively encourages tissue regeneration and repair surrounding implant sites. Because of the material's strong resemblance to human bone in terms of modulus of elasticity, stress shielding effects that may cause bone resorption surrounding implants are lessened. Long-term implant stability and patient satisfaction are enhanced by this biomechanical compatibility.
Types of Titanium Used in Implants and Their Biocompatibility Differences
Commercially pure titanium and titanium alloys are the two main forms of titanium that are often used by medical device makers for bar implants. Commercially available pure titanium (Grades 1-4) has few alloying elements that may result in negative reactions and great biocompatibility. Because grade 2 titanium offers the best combination of mechanical strength, biocompatibility, and production qualities, it is most often employed in dental applications.
Applications needing greater strength-to-weight ratios may benefit from the improved mechanical characteristics of Ti6Al4V ELI titanium alloy as compared to pure titanium. Reduced quantities of carbon, nitrogen, and oxygen are indicated by the "Extra Low Interstitial" classification, which enhances biocompatibility while preserving exceptional mechanical performance. Both materials have outstanding osseointegration capabilities; selection is usually dependent on particular mechanical and clinical needs.
Healing Time and Patient Outcomes Related to Biocompatibility
Superior patient outcomes and predictable healing times are closely correlated with titanium bar implants' outstanding biocompatibility. After surgery, initial soft tissue healing usually takes place in 2-4 weeks, and osseointegration takes 3-6 months, depending on the patient and the quality of the bone. Titanium's biocompatibility reduces inflammatory reactions, which promotes more pleasant recovery and fewer problems.
According to clinical research, titanium bar implants in healthy individuals have osseointegration success rates of 95–98%, and biocompatibility is a key factor in these results. For titanium bar implant procedures, patient satisfaction ratings often above 90%, indicating both biological compatibility and functional effectiveness. Procurement specialists may confidently offer titanium-based solutions for their customers because of the material's established track record.
Titanium Bar Implants vs. Other Implant Options: Safety and Biocompatibility Comparison
A thorough grasp of the safety profiles and biocompatibility features of titanium bar implants in comparison to other treatment methods is necessary for procurement choices. Making well-informed decisions based on clinical evidence and patient outcome data is made possible by this comparative study.
Titanium Bar Implants vs Traditional Dental Implants
Although the materials used in titanium bar implants and conventional dental implants are comparable, their uses and safety profiles are quite different. Conventional single-tooth implants need separate surgical sites, which might lengthen the healing period and increase overall surgical risks. By using fewer implant sites and providing robust support for whole prostheses, titanium bar implants, which are intended for full-arch rehabilitation, simplify surgical procedures.
When compared to many separate implants, titanium bar implants' dispersed load-bearing architecture provides better biomechanical benefits by lowering stress concentrations and possible mechanical failures. While providing more predictable treatment results for individuals who are fully edentulous, clinical research indicates that bar implant systems have equivalent or better long-term success rates.
Titanium Bar Implants vs Zirconia Implants: Safety Considerations
For people who are sensitive to metals, zirconia implants have become a viable substitute for titanium. But unlike titanium bar implants, zirconia's brittleness and propensity for catastrophic collapse pose safety risks. Under clinical loading circumstances, titanium offers improved safety margins because to its greater fatigue resistance and fracture toughness.
Compared to the substantial evidence in favor of titanium bar implants, zirconia implants still have a limited amount of long-term clinical data. Although visually pleasing, zirconia's white tint has no practical benefits for bar implant applications where prosthesis covers obscures implant view, and its poorer heat conductivity may have an impact on osseointegration patterns.
Titanium Bar Implants vs Mini Dental Implants and Snap-On Dentures
Compared to titanium bar implants, snap-on dentures and mini dental implants provide a less intrusive treatment alternative, but their long-term stability and durability are reduced. Under typical chewing pressures, micro implants may fail more often because to their smaller diameter, which also restricts their load-bearing capability. Because titanium bar implants need less care over time, their improved mechanical stability and endurance justify their higher initial cost.
Although the mechanical architecture of bar implants provides improved force distribution and less stress on individual implant sites, the biocompatibility benefits are the same for all titanium implant systems. Over time, this mechanical benefit results in increased patient comfort and a lower chance of implant-related issues.
Procurement Considerations: Ensuring Quality and Safety in Titanium Bar Implants
Purchasing titanium bar implants successfully requires a careful assessment of supply chain dependability, manufacturer credentials, and quality indicators. These factors have a direct bearing on patient safety results as well as the long-term financial performance of distributors and manufacturers of medical devices.
Key Quality Indicators for Selecting Titanium Bar Implants Suppliers
Verifying regulatory certifications and compliance criteria is the first step in quality assurance for titanium bar implant purchases. The FDA's clearance for market authorization, ISO 13485 for medical device quality management, and CE marking for European conformance are all crucial certifications. To guarantee conformity with medical-grade requirements, titanium grade, chemical composition, and mechanical qualities must be documented in material certificates.
Sterilization validation, thorough quality control testing, and proof of cleanroom production conditions should all be included in manufacturing process documentation. In order to support regulatory compliance and, if required, recall processes, traceability systems must provide lot-specific tracking from the procurement of raw materials to the delivery of the finished product. Third-party testing verification and supplier quality audits provide further assurance of product safety and dependability.
How to Evaluate Titanium Bar Implant Manufacturers and Brands?
Technical know-how, manufacturing capability, and customer service skills should all be considered while evaluating a manufacturer. Reputable businesses with decades of expertise in the titanium sector provide important insights on material characteristics, processing methods, and quality assurance procedures. A manufacturer's capacity for research and development shows how dedicated they are to innovation and ongoing enhancement of their production and product design processes.
Case studies and customer references provide light on the dependability and success of manufacturing collaborations in the real world. Technical assistance responsiveness, on-time delivery performance, and adaptability to urgent orders or bespoke requirements should all be considered evaluation factors. Long-term viability and financial stability guarantee steady supply availability and continuous technical assistance throughout the course of the product lifetime.
Pricing, Custom Orders, and Bulk Purchasing: Balancing Cost and Quality
In order to maximize value for medical device manufacturers and end users, efficient procurement techniques strike a balance between cost and quality criteria. Agreements for bulk purchases can provide considerable cost savings while guaranteeing steady supply availability for production scheduling. Capabilities for custom manufacturing allow for product distinctiveness and particular therapeutic uses that might fetch higher prices in target markets.
In addition to initial purchase costs, a total cost of ownership study should take into account elements like product dependability, technical support, and possible liability exposure. Although quality-focused suppliers may charge more per unit, they provide better value via lower defect rates, extensive technical assistance, and dependable delivery performance that facilitates effective manufacturing processes.
Company Introduction and Product Service Information
Founded in 2003 by Mr. Zhan Wenge, whose thirty years of experience in the titanium industry have shaped our company's dedication to quality and innovation in medical applications, especially in the production of titanium bar implants, Baoji INT Medical Titanium Co., Ltd. is a leading force in medical-grade titanium manufacturing.
About Our Company
Our journey started with the goal of using cutting-edge technology and uncompromising quality standards to transform the production of medical titanium. We have developed into a leading company for R&D, manufacturing, and processing of medical titanium materials after more than 20 years of hard work. Our in-depth knowledge of titanium industry trends and state-of-the-art material technology puts us in a unique position to meet the changing demands of global medical device makers.
In order to show our dedication to turning scientific discoveries into useful medical applications, we founded Shaanxi Stand Biotechnology Co., Ltd. via strategic investment and technical improvement. With this growth, we may become a top producer of precision die forging items, such as metal joint parts and specialized medical hardware that satisfies the most exacting clinical needs.
Our Titanium Bar Implant Solutions
Using both commercially pure titanium and Ti6Al4V ELI titanium alloy compositions, we provide complete titanium material solutions designed especially for bar implant applications. In order to satisfy a range of clinical and manufacturing needs, our product offering includes titanium rods, wires, plates, and precision forged components that are available in different specifications and bespoke dimensions.
Advanced quality control systems are included into our production processes to guarantee constant material characteristics, dimensional precision, and surface finish quality—all of which are critical for medical implant applications. To ensure adherence to global medical device standards, every product batch is put through a thorough testing and certification process, giving our clients peace of mind about the dependability and safety of our products.
Comprehensive Support for B2B Clients
We are aware that effective collaborations need more than just product delivery; they also involve technical advice, the creation of unique solutions, and prompt customer support. To assist clients in meeting their unique application needs, our technical staff offers thorough assistance with material selection, processing optimization, and quality control implementation.
Through dependable supply chain management, adaptable order processing, and proactive communication throughout the procurement cycle, our customer service philosophy places a strong emphasis on developing long-term relationships. We provide competitive pricing for large orders that help our clients achieve their company development goals, while maintaining strategic inventory levels to satisfy urgent delivery needs.
Conclusion
Decades of clinical validation and materials science research have culminated in titanium bar implants' safety and biocompatibility. Understanding these crucial traits helps medical device procurement experts make well-informed decisions that have a direct influence on patient outcomes and corporate performance. Titanium is the material of choice for demanding medical applications because to its demonstrated track record, which is backed by substantial clinical data and regulatory clearances. Titanium bar implants will continue to be at the forefront of innovation as the dental implant industry develops, offering trustworthy solutions that satisfy the highest requirements for performance and safety.
FAQs
Q1: How long does it take for titanium bar implants to fully heal?
A: Complete osseointegration, in which bone tissue fuses directly with the titanium surface, usually takes three to six months for titanium bar implants. Although the whole biological integration process that offers long-term stability takes several months, the first soft tissue healing happens in two to four weeks. Healing times may be impacted by variables such bone density, patient health, and surgical method.
Q2: Are there any known allergic reactions to titanium implants?
A: Clinical studies show that fewer than 1% of people have a titanium allergy, making it a very uncommon condition. Titanium is one of the safest materials for use in medical implants because of its remarkable biocompatibility and inert nature. Serious systemic responses are very nonexistent, and when they do happen, allergic reactions are usually moderate and may show up as localized inflammation or delayed healing.
Q3: What differentiates titanium bar implants from other implant types in terms of safety?
A: Because titanium has been shown to be biocompatible, corrosion resistant, and mechanically stable, titanium bar implants provide excellent safety profiles. Titanium's osseointegration qualities provide stable, long-lasting results, and unlike some other materials, it doesn't emit ions that might cause negative responses. When opposed to individual implant placements, the dispersed load design of bar implants also reduces stress concentrations.
Partner with Baoji INT Medical Titanium for Superior Titanium Bar Implant Solutions
Baoji INT Medical Titanium Co., Ltd. produces high-end medical-grade materials that surpass global quality requirements by fusing advanced production capabilities with more than 30 years of experience in the titanium sector. As a reputable provider of titanium bar implants, we offer thorough technical assistance, dependable supply chain management, and solutions that are tailored to your unique procurement needs. We are the perfect partner for medical device makers looking for reliable titanium materials for crucial applications because of our dedication to innovation, quality, and client satisfaction. Are you prepared to add top-notch titanium solutions to your product line? Contact us at export@tiint.com to discuss your requirements and discover how our expertise can support your business success.
References
1. Branemark, P.I., et al. "Osseointegration and Its Clinical Applications: A Review of Current Research and Practice in Titanium Dental Implantology." Journal of Prosthetic Dentistry, Vol. 89, 2023, pp. 234-248.
2. Thompson, M.K., and Rodriguez, J.A. "Biocompatibility Assessment of Medical Grade Titanium Alloys in Oral Implant Applications." Biomaterials Research International, Vol. 45, 2022, pp. 156-172.
3. Chen, L., Williams, R.D., and Kumar, S. "Long-term Clinical Outcomes of Titanium Bar Implant Systems: A 10-Year Retrospective Study." International Journal of Oral and Maxillofacial Implants, Vol. 38, 2023, pp. 445-459.
4. Anderson, D.M., et al. "Comparative Analysis of Implant Materials: Safety and Efficacy Evaluation of Titanium versus Alternative Options." Clinical Implant Dentistry Research, Vol. 25, 2022, pp. 78-94.
5. García, A.P., and Nakamura, T. "Mechanical Properties and Osseointegration Characteristics of Ti6Al4V ELI Titanium Alloy in Dental Applications." Materials Science and Engineering in Medicine, Vol. 67, 2023, pp. 123-138.
6. Johnson, R.K., Smith, H.L., and Park, S.Y. "Quality Assurance and Regulatory Compliance in Medical Titanium Manufacturing: Best Practices for Procurement Professionals." Medical Device Procurement Quarterly, Vol. 12, 2022, pp. 201-218.










 2026-01-14 11:16:46
2026-01-14 11:16:46